Intermediate Uveitis
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Intermediate uveitis (IU) is a chronic, relapsing disease of insidious onset. According to the Standardization of Uveitis Nomenclature (SUN) working group criteria, IU is defined as an intraocular inflammation mainly focused on the vitreous and peripheral retina. It incorporates pars planitis, posterior cyclitis, and hyalitis.
IU accounts for 1.4 to 31% of all uveitis patients, and up to 20% of cases of pediatric uveitis.
Epidemiology
IU is a rare disease, with an estimated incidence of 1.4-2.0 /100.000. In 2004, a large-scale study tracking incidence and prevalence of uveitis in Northern California showed that in intermediate uveitis, there was an incidence of 1.5/100,000 person-years and a period prevalence of 4.0/100,000 persons. These values were notably higher in patients 45-64, with lower incidence and prevalence in pediatric patients and females.
The annual incidence of par planitis was 2.077 per 100,000 individuals in a 20-year study evaluating patients in Olmsted County, Minnesota. Within various pediatric populations, pars planitis is responsible for 5 to 26.7% of uveitis cases.
However, the epidemiology of IU seems to have geographical differences, possibly due to different etiologies. Whereas in Europe, the United States and China IU is usually idiopathic, in other parts of the world, such as India, infectious causes, particularly tuberculosis, are more frequent.
Risk Factors:
- Race and ethnicity: Non-Hispanic, Caucasian
- Approximately equal gender distribution in pediatric populations; slight female preponderance in older cases
- Median age of onset: 8-11 years
- Genetics: HLA-DR2 and its split antigen HLA-DR15
Etiology
General IU may be due to a number of causes. However, the exact pathophysiology of pars planitis remains unclear. It appears to be an immunologically mediated condition involving a CD4+ T-cell mediated response to an endogenous antigen of unknown origin. This reaction leads to inflammation in the vitreous and peripheral vasculitis. Analysis of aqueous humor and peripheral blood in pars planitis patients has shown that CD4+ T cells expressing the activation marker CD69 are present. Elevated IL-6 levels have been observed in the vitreous of patients with active intermediate and posterior uveitis, supporting the involvement of autoimmunity in the manifestation of the disease.
Infectious causes
- Tuberculosis
- Leprosy
- Lyme’s disease
- Syphilis
- Toxocariasis
- Whipple’s disease.
Non-infectious causes
- Sarcoidosis – about 25% of patients with sarcoidosis develop IU, and 2 to 10% of patients with IU develop sarcoid disease
- Blau syndrome
- Multiple sclerosis (MS) – the proportion of MS in IU patients varies from 7 to 30.4%, and 3-27% of patients with MS develop IU. IU in children is rarely related to MS, but children with IU may develop MS later in life. Retinal periphlebitis at the time of diagnosis increased the risk of MS and/or optic neuritis. Associations identified between the condition and HLA‑DR2, ‑DR15, ‑B51, and ‑DRB1 × 0802 haplotypes suggest an immunogenetic predisposition.
- Inflammatory Bowel Disease
- Lymphoma – 66% of intraocular lymphomas are a manifestation of a primary central nervous system lymphomas (PCNSL), and 10-20% of them may present as vitreous or retinal infiltrates mimicking uveitis.
- Tubulointerstitial nephritis and uveitis (TINU) syndrome
- Sjogren syndrome.
Pars Planitis (Idiopathic IU)
Pars planitis is an idiopathic subtype of intermediate uveitis characterized by fibroinflammatory aggregates at the pars plana (“snowbanks”) and peripheral retina (“snowballs”). It is a diagnosis of exclusion that most commonly affects the pediatric patient population. The condition is typically bilateral, yet asymmetric, with more severe cases observed in those with an earlier age of onset. Common findings include mild to moderate anterior segment inflammation, diffuse vitreous cells, vitreous haze, sheathing of peripheral retinal venules, and retinoschisis.
General Pathology
Histologic studies show condensed vitreous, fibroblasts, lymphocytes, and lymphocyte cuffing of the peripheral retinal veins. Snowbanks and snowballs consist of a fibrovascular layer containing mononuclear leukocytes and fibrocyte-like cells, as well as vitreous collagen, Muller cells and fibrous astrocytes.
Histologic examination of eye tissue affected by pars planitis reveal condensed vitreous, fibroblasts, lymphocytes, and lymphocyte cuffing around the peripheral retinal veins. The formations known as snow banks and snowballs are composed of a fibrovascular layer containing mononuclear leukocytes and fibrocyte-like cells, in addition to vitreous collagen, Müller cells, and fibrous astrocytes.
Pathophysiology
The exact pathophysiology of intermediate uveitis in general remains unclear. However, it appears to be an immunologically mediated condition involving a CD4+ T-cell mediated response to an endogenous antigen of unknown origin; these cells account for up to 95% of all cell types in the vitreous of patients with IU. This reaction leads to inflammation in the vitreous and peripheral vasculitis. Analysis of aqueous humor and peripheral blood in pars planitis patients has shown that CD4+ T cells expressing the activation marker CD69 are present. Elevated IL-6 levels have been observed in the vitreous of patients with active intermediate and posterior uveitis, supporting the involvement of autoimmunity in the manifestation of the disease.
Genetic factors also play a role in pars planitis pathogenesis. Associations identified between the condition and HLA‑DR2, ‑DR15, ‑B51, and ‑DRB1 × 0802 haplotypes suggest an immunogenetic predisposition. Other HLA‑DR15-related disorders include multiple sclerosis, optic neuritis, and narcolepsy, indicating a shared genetic background with pars planitis. HLA-DR is the most significant occurring in 67-72% of patients.
Clinical Manifestations
History
IU classically affects younger patients, aged 15 to 40 years. Reported symptoms of pars planitis are usually unilateral with gradual onset beginning with mild vision blurring and floaters. Severe vitreous inflammation in pars planitis is often associated with uveitic macular edema, which may lead to decreased vision. Rarely, intravitreous hemorrhage due to neovascularization may impair vision in patients with intermediate uveitis. Ocular symptoms may be mild or absent, with intermediate uveitis being incidentally noted on routine examination.
Symptoms
- Painless floaters
- Decreased/blurred vision
- Pain, photophobia and red eye are rare symptoms
Physical Examination
Anterior Segment Findings
The frequency of common examination findings in patients with pars planitis include:
- Anterior chamber cells:
- Grade 0: 19.5%
- Grade ½+: 11.9%
- Grade 1+: 8.4%
- Grade 2+: 4.0%
- Grade 3+: 0.4%
- Anterior chamber flare:
- Grade 0: 33.2%
- Grade 1+: 9.3%
- Grade 2+: 1.3%
- Grade 3+: 0.4%
- Small keratic precipitates:
- None: 36.7%
- Fine: 6.6%
- Round: 0.9%
Posterior synechiae, particularly involving the inferior iris: 5.3%
Peripheral corneal endotheliopathy (Figure 1), evidenced by opacification of the posterior cornea in the inferior region: 17.7%
Other anterior segment findings:
- Linearly arranged keratic precipitates at the junction of the edematous and nonedematous cornea suggest an underlying autoimmune disease, akin to the phenomenon of corneal allograft rejection.
- Band keratopathy, a deposition of calcium in the cornea, has been documented in children with IU, a condition that may arise in various chronic pediatric uveitis cases. Possible inferior posterior corneal haze may be present in fundoscopic exam due to inflammatory endotheliopathy.
Posterior Segment Findings
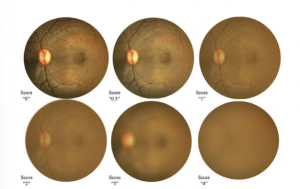
- Pars planitis is a subtype of intermediate uveitis. Intermediate uveitis is characterized by inflammation of the vitreous, which manifests with vitreous cells and vitreous haze. Intermediate uveitis activity grading is based on vitreous haze, utilizing Nussenblatt scale (Table 1, Figure 2a), additional reference of vitreous haze in Figure 2b. In severe cases of pars planitis, the density of vitreous inflammation may obscure the retina, posing challenges in excluding a diagnosis of posterior uveitis
- Vitreous snowballs, recognizable as globular yellow-white inflammatory clusters, are commonly found in the mid vitreous and inferior periphery (Figure 3). While situated close to the retina, they do not make direct contact and have been reported in up to 100% of eyes affected with pars planitis.
- Snowbanking is the distinctive hallmark of pars planitis (Figure 4). These white or yellowish-white formations typically span the pars plana, originating inferiorly and progressing superiorly.
- Degenerative changes in the vitreous, marked by fiber-like cylindric condensations of coarse vitreous strands, may be observed.
- Peripheral retinal vascular sheathing, primarily affecting venules, is a frequent occurrence, seen in 10–32% of patients (Figure 4).
- Peripheral periphlebitis is a common finding (16-36%), particularly in patients with MS
- Younger patients may present with vitreous hemorrhage.
Diagnosis
IU is typically a clinical diagnosis and requires exclusion of infectious and associated systemic inflammatory disease. Evaluation for exclusion involves testing for infectious etiologies such as tuberculosis, syphilis, and Lyme disease, as well as autoimmune mediated etiologies such as sarcoidosis, multiple sclerosis, and Behçet disease.
Notably, malignancy should be considered and investigated in elderly patients, as primary intraocular lymphoma may present with diffuse vitritis mimicking IU. Along with severe vitritis, chorioretinal lesions and poor or partial response to therapy should raise suspicion for lymphoma.
The Standardization of Uveitis Nomenclature (SUN) Working Group has provided classification criteria for distinct uveitides including pars planitis for research as well as clinical use (Table 2).
| Name | Etiology | Classification | Exclusion |
| Pars planitis | Eye-Limited |
AND
|
|
Table 2. SUN II Classification of pars planitis. Adapted from EyeWiki: http://eyewiki.org/SUN_II_Classification_of_Uveitides#SUN_II_Classification_of_Uveitides
Laboratory studies
- Complete blood count (CBC)
- Erythrocyte sedimentation rate (ESR) and or C-reactive protein (CRP)
- Angiotensin-converting enzyme (ACE)
- Lysozyme
- Non-treponemal and treponemal testing (RPR or VDRL and FTA-ABS)
- Interferon gamma release assay (IGRA)
- Tuberculin skin test
Imaging
- Chest radiograph and/or chest computerized tomography (CT)
- Brain, orbit, and spinal magnetic resonance imaging (MRI)
Ocular imaging
Digital color fundus photography
In patients with IU, fundus photographs allow documentation of baseline appearance of retinal lesions, and helps to assess progression of the condition and response to treatment during follow up. Peripheral retinal pathologies such as snowbanking, retinoschisis, peripheral tractional membranes and macular complications such as macular edema, epiretinal membranes, atrophy and scars can be documented by color fundus photography.
Optical coherence tomography (OCT)
OCT imaging is very useful in evaluating the macula for CMO, ERM, macular hole ad atrophy. It can be a useful tool for the follow-up of these patients, and allows monitoring the response to treatment. Visual potential and prognostic factors can also be determined by OCT: correlations have been shown between:
- vision and foveal thickness
- IS/OS junction abnormalities and poorer visual prognosis
- CMO with healthy IS/OS junction and better visual prognosis with aggressive treatment.
Intravenous Fluorescein Angiography (IVFA)
IVFA is beneficial to show the activity of retinal vascular inflammation in IU. It allows the detection of CMO, and it helps detect retinal vasculitis in the posterior segment (Figure 5) and may demonstrate areas of retinal nonperfusion and neovascularization
Indocyanine green angiography (ICGA)
ICGA has not demonstrated any clinical usefulness in cases of IU
Ultrasonography (US)
US imaging may be helpful in cases where media opacities (band keratopathy, cataract, or significant vitreous inflammation) preclude adequate visualization of the fundus and do not allow for OCT imaging. US at 20- and 50-Hz frequencies can detect snowbanking in IU, and 20-Hz probe may be more useful to detect the presence of CMO.
Ultrasound biomicroscopy
Ultrasound biomicroscopy (UBM) supplements ocular examination and can be used to evaluate patients with IU. UBM utilizes frequencies between 35–100 MHz, offers detailed images of the pars plana region, detecting features such as snowbanking, cyclitic membranes, peripheral vitreous membranes, and vitreoretinal traction. It also serves as a valuable tool for monitoring treatment response
Diagnostic Procedures
Diagnostic vitrectomy
Diagnostic vitrectomy may be necessary in:
- Suspicion of malignancy,
- Cases where retinitis or endophthalmitis cannot be excluded,
- Cases refractory to medical therapy.
Samples can be obtained by vitreous aspiration, vitreous biopsy, or chorioretinal biopsy.
- Vitreous aspiration (0.2-0.5 cc are aspirated using a 23—gauge needle) is associated with more traction at the vitreous base, and increased risk of hypotony and retinal detachment.
- Vitreous biopsy (1 cc of vitreous is collected directly from the vitrectomy bag) is associated with lesser vitreous traction and can be combined with therapeutic vitrectomy.
- Chorioretinal biopsies are highly invasive, and are only indicated in cases where other techniques have failed and disease is bilateral and rapidly progressive. It is usually performed in the worst-seeing eye, and can be done via a scleral flap or by pars plana vitrectomy.
Differential Diagnosis
- Other intermediate uveitides including:
- Syphilitic uveitis
- Ocular tuberculosis
- Toxocariasis
- Lyme-associated uveitis
- Sarcoidosis
- Multiple sclerosis
- Behçet uveitis
- Fuchs uveitis
- Masquerades such as:
- Vitreoretinal lymphoma
- Retinoblastoma
Management
Treatment for pars planitis is warranted in cases of reduced vision, substantial vitreous opacities, macular edema, retinal vasculitis, or other uveitic complications. Various approaches include the use of topical, periocular, systemic, and intravitreal corticosteroids. In instances of multiple recurrences in the affected eye(s), chronic uveitis, steroid-induced ocular hypertension/glaucoma, or corticosteroid-refractory inflammation, treatment escalation is advised. Options for treatment escalation most commonly involve steroid sparing immunomodulatory therapy, but also may include peripheral retinal cryopexy, indirect laser photocoagulation, or pars plana vitrectomy. Malignancy and infection must be ruled out before commencing nonspecific anti-inflammatory therapy.
- Mild vitreous cells in the absence of symptoms or vision loss may be observed. However, little consensus exists as to what threshold to adopt in these cases
- Treat vision-threatening complications (CMO, vitritis) in symptomatic patients with active disease.
Some 25% to 35% of patients with pars planitis have mild disease, no macular edema or other complications, and good vision, and do not need treatment; these patients maintain good vision with up to 10 years of follow-up.
However, when treatment is needed, the goal is complete suppression of the inflammation (i.e., to “grade 0” inflammation).
A stepladder approach has been the most widely adopted strategy in the treatment of IU, with CS therapy being the mainstay of treatment:
Corticosteroids
Periocular CS
Periocular CS (e.g., subtenon injection of 0.5-1.0 mL of triamcinolone acetonide 40 mg/mL)are beneficial in patients with unilateral or asymmetric involvement and in the presence of macular edema. Injections can be repeated every 6 to 8 weeks until the vision and CMO have stabilized; at least 2 or 3 injections are suggested before considering this modality ineffective.
Intravitreal steroids (IVS)
Triamcinolone acetonide (IVTA)
IVTA is associated with high rates of complications, and thus many authors consider it an emergency procedure when essential structures such as the macular have to be rescued immediately, to allow time to organize the long term management.
Slow-release intravitreal implants
Slow-release dexamethasone 0.7 mg intravitreal implant (Ozurdex®; Allergan, Irvine, CA, USA)
Ozurdex has been approved for treatment of intermediate and posterior uveitis, after having shown in case series to significantly improve retinal thickness and visual acuity and to delay and reduce recurrence of uveitic CMO.
Sustained-release fluocinolone acetonide 0.19 mg (Iluvien®, Alimera) or 0.59 mg intravitreal implant Retisert®, Bausch and Lomb)
The MUST trial: compared systemic anti- inflammatory therapy vs. fluocinolone acetonide intraocular implant for intermediate uveitis, posterior uveitis, and panuveitis. No difference was observed in the rates of improvement in macular edema; however, subjects treated with fluocinolone implant had greater quantitative improvement in macular thickness over the follow-up period. A few studies have shown implanted eyes have improved CMO (Pavesio et al, Callanan et al). The use of fluocinolone implants has been limited by the high rate of complications, including cataract formation, ocular hypertension, and hypotony.
Systemic CS
Patients with bilateral disease, severe ocular inflammation, or unilateral disease unresponsive to periocular steroids should be treated systemically, e.g., prednisone 1.0-1.5 mg/ kq body weight/day per os for 4-6 weeks, with gradual tapering according to patient’s response.
Steroid-sparing immunosuppressive therapy
Immunosuppressants are considered a second step in patients for long-term treatment. Indications for commencing systemic immunomodulatory therapy are:
Ocular and anatomical
- Acute, vision-threatening disease
- Chronic, persistent disease
- Exudative RD
- Macular involvement
- Bilateral, vision-threatening disease
Therapeutic failure
Failure of local steroid therapy
Failure of systemic steroid therapy
- Active uveitis with doses of prednisone 30 mg or 0.5 mg/kg/day
- Recurrence of uveitis after tapering oral CS to less than 7-10 mg/day
- Intolerance to oral CS
- Need for steroid-sparing.
The choice of immunosuppressive agent is dependent on the patient’s clinical findings, patient comorbidities, and on the ophthalmologist’s preference and experience.
Methotrexate is the most widely used first‐line immunosuppressive agent in children with chronic non-infectious uveitis because of its long-term safety record and well tolerance. Cyclosporine has also been reported effective. Other drugs used include , mycophenolate mofetil, azathioprine, leflunomide, tacrolimus and rituximab.
Anti-tumor necrosis factor- α (Anti-TNF-α) agents
These class of agents include adalimumab and infliximab. Anti-TNF-α agents may be used successfully as the third step in patients not responding to conventional immunosuppressive agents, although no study exists directly addressing the use of these drugs in non-infectious IU.
However, since pars planitis is associated with increased risk for MS development, Anti-TNF-α agents should be employed with extreme caution, as they may potentiate demyelinating disease.
Interferon α may help improve macular oedema in non-infectious IU, and interferon β has showed success in the treatment of IU-related CMO. However, to date no study has directly compared Interferon α versus interferon β for this indication.
Pars plana vitrectomy (PPV)
For cases unresponsive to medical intervention, surgical options should be considered for further treatment. In cases where complications such as vitreous condensation, vitreous hemorrhage, retinal detachment, and issues with epiretinal membranes causing retinal traction or macular hole, pars plana vitrectomy (PPV) is a viable surgical intervention. PPV has been used in the following clinical scenarios:
- Vitreous condensations
- Vitreous hemorrhage
- Retinal detachment
- ERM causing retinal traction
- Ciliary traction causing hypotony
- Active inflammation and CMO refractory to medical treatment.
Vitrectomy promotes mechanical clearance of inflammatory mediators and debris, and anatomical correction of vitreoretinal traction. In addition, it may be used for diagnostic purposes.
Cryotherapy and laser photocoagulation
Although cryotherapy was used to induce regression of vitreous base neovascularization and to stabilize inflammation, it has now fallen out of favor, as it might aggravate blood-ocular-barrier disruption and possibly accelerate the rate of retinal detachment.
Laser photocoagulation is currently considered as an adjunctive approach to other treatment modalities, especially in cases associated with peripheral neovascularization, retinal traction, or retinoschisis.
Medical follow up
In the acute phase, patients are reevaluated every 1 to 4 weeks, depending on disease severity.
In the chronic phase, reexamination is performed every 3 to 6 months.
Complications
IU can lead to blindness due to complications and permanent damage to ocular structures, especially if the diagnosis is delayed. Children are at high risk of amblyopia.
- Neovascularization in the peripheral retina (6.5%), usually associated with snowbanking
- Neovascularization in the optic nerve head
- Band keratopathy (in up to 45% of eyes)
- Optic disc swelling (3 to 7.4%)
- Cystoid macular oedema (CMO) is a common complication of IU, occurring in up to 50% of patients, and is the major cause of impaired vsion in these patients. The risk of CMO increases with disease severity and duration
- Epiretinal membrane (ERM) formation is common, occurring in approximately 35% of eyes
- Cataract formation in 15-50% of eyes, usually PSC or anterior subcapsular
Cataract surgery in patients with IU should only be performed after inflammation has cleared, ideally with no inflammatory for at least 3 months.
Some authors advocate starting oral prednisone 1 mg/kg body weight/day for 5 days before surgery, with tapering over the following month.
An adequate preoperative control of inflammation, a meticulous surgical technique, a foldable hydrophobic acrylic intraocular lens implanted in the capsular bag and good postoperative inflammation control are crucial for successful cataract surgery in pars planitis patients. A combined pars plana vitrectomy may be considered in cases with significant vitreous opacity.
Studies have shown good clinical outcomes after cataract surgery in patients with IU, with 90% of patients obtaining at least some improvement in vision, and 88% of patients achieving visual acuity above 20/40.
- Uveitic glaucoma occurs in 7.6% of acute uveitis cases and in 11.1% of patients with chronic IU at 5 years
- Retinal detachment (RD, 2.2-51% of eyes): all forms of RD may occur, including exudative RD secondary to inflammation, tractional due to vitreous traction, and rhegmatogenous secondary to vitreous traction leading to peripheral hole formation
- Inferior peripheral retinoschisis is a complication which occurs almost exclusively in children.
Prognosis
The natural history of IU is variable. Although generally considered a benign disease, many cases show a prolonged course with exacerbations, and only 10% of patients may have a self-limited course of disease. In addition, many patients develop complications that may lead to visual loss. Adequate control of inflammation and prompt detection and treatment of associated complications are thus essential in improving the overall prognosis of the patient.
Overall, the visual prognosis of pars planitis in children is reported to be favorable if adequate treatment is implemented early in the disease course. In a study conducted by Donaldson, et. al., of 24 patient eyes, 75% maintained a visual acuity of 20/40 or better after 10 years of follow-up.
Severe visual loss is uncommon, but there is a high incidence of ocular complications, such as optic disc edema leading to optic atrophy and macular edema, which are associated with visual impairment. Long-term follow-up suggests that although optic disc edema may indicate a risk for macular edema, it is noted that not all patients with optic disc edema develop substantial visual loss.
Factors associated with better outcomes in pars planitis include older age of disease onset and prompt initiation of systemic steroid-sparing IMT. Ongoing monitoring is advised to assess for uveitic and steroid-induced complications such as cataracts or glaucoma.
References
1. Brad Bowling. Kanski's Clinical Ophthalmology - A Systematic Approach, 8th edition. Elsevier , 2016.
2. Myron Yanoff, Jay S. Duker. Ophthalmology, 4th edition. Elsevier, 2014.
3. Nika Bagheri, Brynn N. Wajda. Will's Eye Manual - Office and Emergency Room Diagnosis and Treatment of Eye Disease, 7th edition Wolters Kluwer. 2017.
4. Pinar Cakar Ozdal, Nilufer Berker, Ilknur Tugal-Tutkun. Pars Planitis: Epidemiology, Clinical Characteristics, Management and Visual Prognosis. J Ophthalmic Vis Res. 2015 Oct-Dec; 10(4): 469–480. doi: 10.4103/2008-322X.176897
5. Thomas Ness, Daniel Boehringer, Sonja Heinzelmann. Intermediate uveitis: pattern of etiology, complications, treatment and outcome in a tertiary academic center. Orphanet J Rare Dis. 2017; 12: 81. doi: 10.1186/s13023-017-0638-9.
6. B Manohar Babu, S R Rathinam. Intermediate uveitis. Indian J Ophthalmol. 2010 Jan-Feb; 58(1): 21–27. doi: 10.4103/0301-4738.58469.
7. Douglas A Jabs. Immunosuppression for the Uveitides. Ophthalmology. 2018 Feb;125(2):193-202. doi: 10.1016/j.ophtha.2017.08.00.
8. Gritz D.C. & Wong I.G. (2004). Incidence and prevalence of uveitis in Northern California; the Northern California Epidemiology of Uveitis Study. Ophthalmology, 111(3):491-500. doi.org/10.1016/j.ophtha.2003.06.014
9. Donaldson M.J, Pulido J.S., Herman D.C., Diehl N., & Hodge D. (2007). Pars planitis: a 20-year study of incidence, clinical features, and outcomes. American Journal of Ophthalmology, 144(6):812-817. doi:10.1016/j.ajo.2007.08.023
10. Nguyen, A.T., Koné-Paut, I. & Dusser, P. (2024). Diagnosis and Management of Non-Infectious Uveitis in Pediatric Patients. Pediatric Drugs, 26:31-47. doi:10.1007/s40272-023-00596-5
11. Khochtali, S., Ozdal, P., et al. (2023). Pediatric Pars Planitis: A Review. Ocular Immunology and Inflammation, 31(10):1915-1929. doi:10.1080/09273948.2023.2279683
12. Jabs, D.A., Denniston, A.K., et al. (2021). Classification Criteria For Pars Planitis. American Journal of Ophthalmology, 228:268-274. doi:10.1016/j.ajo.2021.03.045
13. Hunchangsith, Boonsiri. (2020). Pars Planitis. Uveitis: A quick guide to essential diagnosis, 49-52. doi:10.1007/978-3-030-52974-1_11
14. Ozdemir, H.B. & Ozdal, P.C. (2022). Clinical characteristics and treatment of pars planitis: an adalimumab experience. Graefe's Archive for Clinical and Experimental Ophthalmology, 260:561–569. doi:10.1007/s00417-021-05398-4
15. Navarrete, A., Koriat, A. & Amer, R. (2020). Implications of pars planitis-associated cystoid macular edema on visual outcome and management in children. Graefe's Archive for Clinical and Experimental Ophthalmology, 258:1803–1811. doi:10.1007/s00417-020-04696-7
16. Kosmaczewska, A, Przeździecka-Dołyk, J, et al. (2020). Imbalance in PB IL-17-Secreting and Regulatory Cells in Pars Planitis Is Associated with Dysregulation of IFN-γ-Secreting Cells, Especially in Patients with Clinical Complications. Mediators of Inflammation, 2020:1-9. doi:10.1155/2020/9175083
17. Agarwal, A., Aggarwal, K., & Gupta, V. (2019). Pars Planitis (Idiopathic Intermediate Uveitis of the Pars Planitis Type). The Uveitis Atlas, 475-478. doi:10.1007/978-81-322-2410-5_88
18. Chauhan K. & Tripathy K. (2023). Pars Planitis. StatPearls, available from: http://www.ncbi.nlm.nih.gov/books/NBK436019/
19. Bahar, Merve. (2023). Pars Planitis Epidemiology, Diagnosis, Follow-Up And Prognosis. Cumhuriyet Medical Journal, 45(4): 8-16. doi.org/10.7197/cmj.1386749
20. Arellanes García, L., Ortiz-Ponce, G., & Recillas-Gispert, C. (1996). Peripheral corneal endotheliopathy and pars planitis. Ocular immunology and inflammation, 4(3), 135–138. doi.org/10.3109/09273949609079646
21. Thomas, A.S., Amro, A, Sruthi, A, & Suhler, E. B. (2020). Inferior Corneal Haze and Inflammatory Endotheliopathy Related to Pars Planitis. Ocular Immunology and Inflammation, 28(5):798-801. doi:10.1080/09273948.2019.1629603
22. Group SoUNSW. Classification Criteria For Pars Planitis. (2021). American Journal of Ophthalmology, 228:268-274. doi:10.1016/j.ajo.2021.03.045
23. Concha del Río, L., Duarte González, G., Mayorquín Ruiz, M. et al. (2020). Characterization of cyclitic membranes by ultrabiomicroscopy in patients with pars planitis. Journal of Ophthalmic Inflammation and Infection, 10:7. doi:10.1186/s12348-020-0194-7
24. Yalçındağ, F.N., Temel, E. & Özgür, E.G. (2021). Spectral domain optical coherence tomography findings of patients with pars planitis and risk factors affecting visual acuity. International Ophthalmology, 41:1753–1761. doi:10.1007/s10792-021-01734-z
25. Faia, Lisa J. (2023). Pars Planitis in Children. Pediatric Vitreoretinal Surgery, 765-777. doi:10.1007/978-3-031-14506-3_53
26. Ozer, M.D., & Batur, M. (2023). Anti-tumor necrosis factor-alpha treatment in pediatric patients with pars planitis: a single-center experience from Turkey. International Ophthalmology, 43:155–166. doi:10.1007/s10792-022-02398-z
27. Rosenbaum, J.T., Bodaghi, B, et al. (2019). New observations and emerging ideas in diagnosis and management of non-infectious uveitis: A review. Seminars in Arthritis and Rheumatism, 49(3):438-445. doi:10.1016/j.semarthrit.2019.06.004
28. de Boer, J., Berendschot, T. T., van der Does, P., & Rothova, A. (2006). Long-term follow-up of intermediate uveitis in children. American journal of ophthalmology, 141(4), 616–621. doi.org/10.1016/j.ajo.2005.09.035